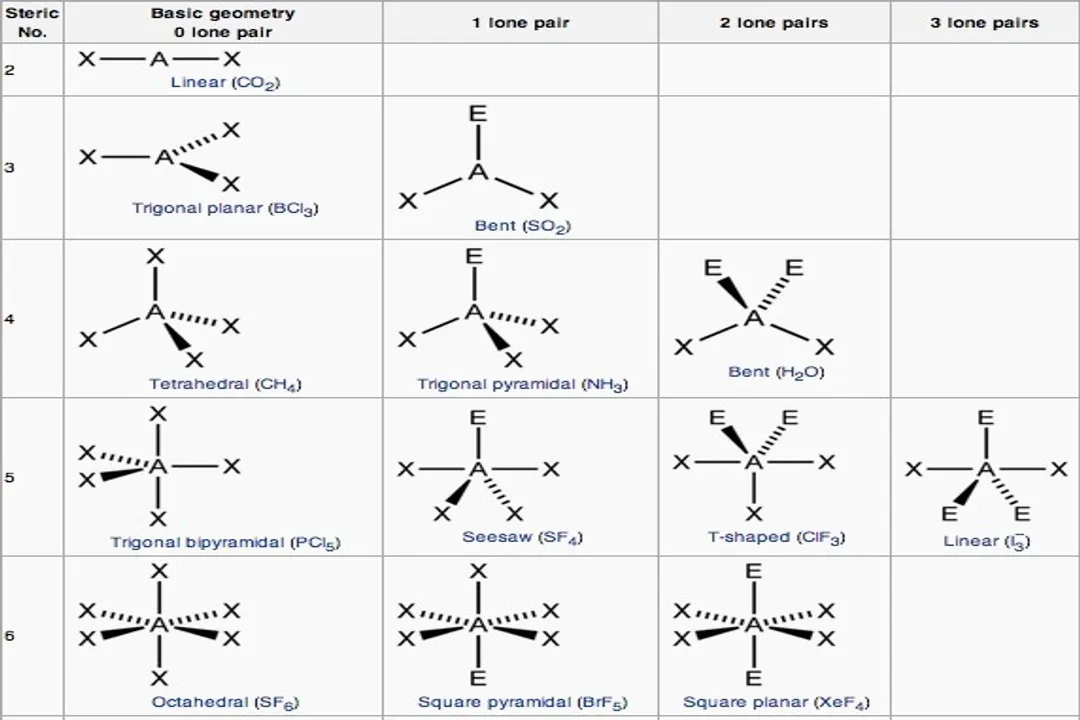
What is the order of repulsion strength between electron domains, from greatest to least?
Answer
Lone Pair - Lone Pair > Lone Pair - Bonding Pair > Bonding Pair - Bonding Pair
The repulsion between two lone pairs is the strongest, followed by repulsion between a lone pair and a bonding pair, with repulsion between two bonding pairs being the weakest.
Related Questions
What constitutes an electron domain in the VSEPR model?For the purpose of determining initial geometry, how is a triple bond counted regarding electron domains?What is the order of repulsion strength between electron domains, from greatest to least?Why do lone pairs exert stronger repulsion than bonding pairs?What molecular geometry results when a molecule has a tetrahedral electron geometry with two bonding pairs and two lone pairs ($ ext{AX}_2 ext{E}_2$)?What is the electron geometry when a molecule has five total electron domains?What molecular geometry is characteristic of a molecule with five domains where one domain is a lone pair ($ ext{AX}_4 ext{E}$)?In an Octahedral electron geometry (6 domains), what shape is formed if there are two lone pairs ($ ext{AX}_4 ext{E}_2$)?What is the approximate bond angle found in water ($ ext{H}_2 ext{O}$), which has two lone pairs on the central oxygen atom?Which factor causes the slight deviation of bond angles from the idealized VSEPR angles in real molecules like chloromethane?