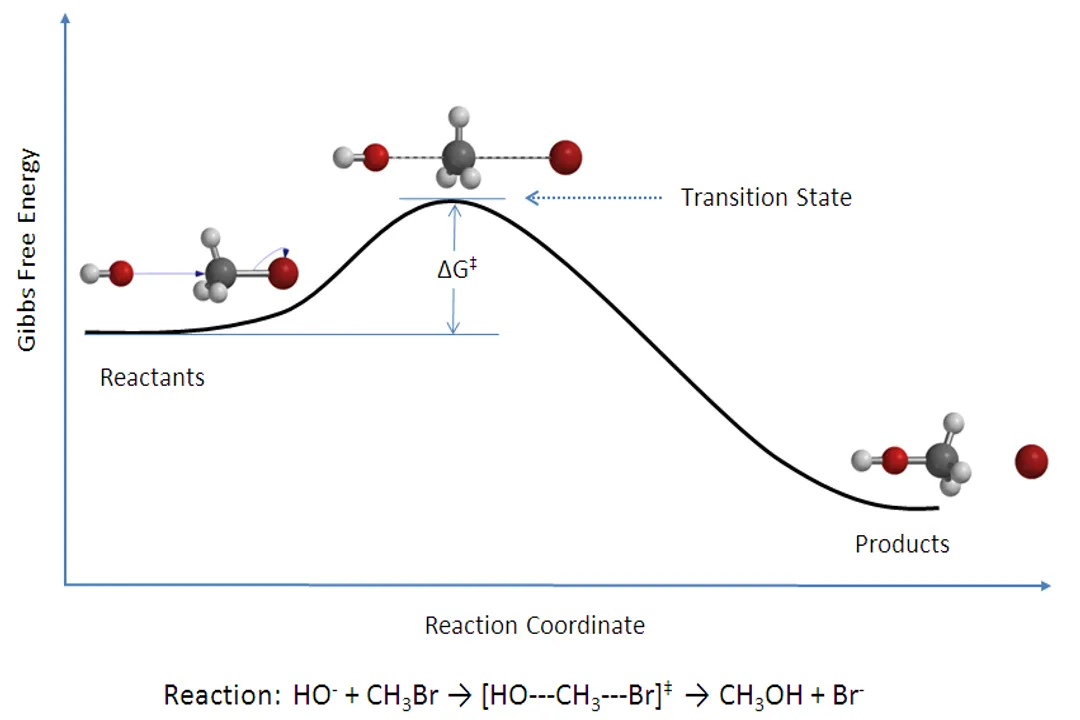
When optimizing a synthesis, why might increasing the reaction temperature decrease the yield of the desired product?
Answer
If competing side reactions have an even higher activation energy, the temperature increase disproportionately favors their formation.
The optimum temperature must balance speed and selectivity. If increasing temperature accelerates an unwanted side reaction more rapidly than the desired main reaction (due to differences in $E_a$), selectivity is lost, lowering the yield of the target product.
#Videos
How does temperature affect rate of reaction? - YouTube
Related Questions
What determines if a molecular collision leads to a successful chemical reaction?What is the dominant molecular factor causing reaction rates to increase significantly with rising temperature?What quantity is represented by the slope ($m$) when plotting $\ln(k)$ against $1/T$ in the linearized Arrhenius equation?In a multi-step reaction mechanism, which step controls the overall observed rate?How is the term $e^{-E_a / RT}$ defined within the context of the Arrhenius Equation?Why are reactions with very high activation energies naturally slow at room temperature?What consequence results when biological enzymes are subjected to temperatures significantly above their optimum?How does the sensitivity to temperature changes compare between a high $E_a$ reaction and a low $E_a$ reaction?In the context of shelf-life testing, how do chemists use the Arrhenius relationship?When optimizing a synthesis, why might increasing the reaction temperature decrease the yield of the desired product?