Which of the following represents an irreversible process causing real engines to fall short of the Carnot limit?
Answer
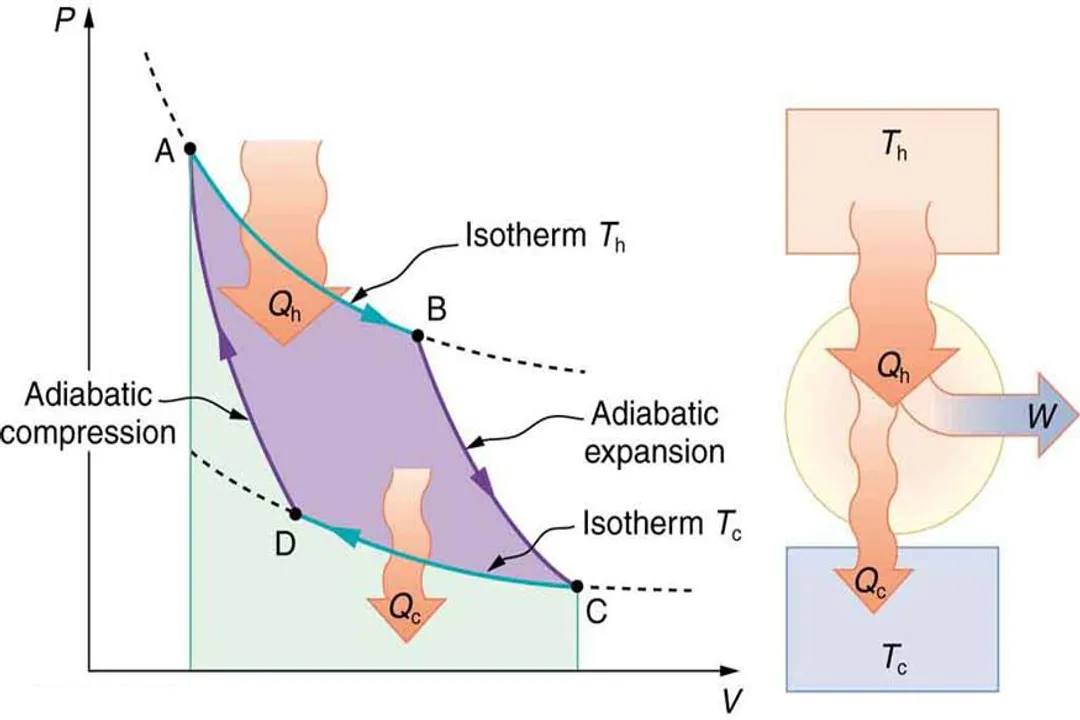
Heat transfer occurring across a finite, non-zero temperature difference between components (e.g., combustion gas and working fluid).
In real cycles, heat must be transferred across a non-zero temperature gap to achieve a useful rate of energy transfer, which represents an inherent, irreversible loss of potential efficiency compared to the idealized Carnot process.
#Videos
The fundamental limits of efficiency for quantum heat engines
Related Questions
How is the thermal efficiency ($ ext{eta}$) of a heat engine calculated based on energy inputs and outputs?What fundamental requirement dictates that the efficiency of any real heat engine must always be less than 100%?What factors exclusively determine the Carnot efficiency, according to the derived formula?In what temperature scale must $T_H$ and $T_C$ be expressed to correctly calculate Carnot efficiency?What is the fundamental thermodynamic requirement for extracting work from a heat engine?Which principle serves as the theoretical foundation proving that no heat engine can be more efficient than a reversible engine operating between the same two temperatures?Which of the following represents an irreversible process causing real engines to fall short of the Carnot limit?Besides thermodynamic limits, what practical constraint governs the achievable operating temperature ($T_H$) in modern engines?How does the Second Law of Thermodynamics differentiate itself from the First Law regarding energy?If Plant A has $T_H = 900 ext{ K}$ and Plant B has $T_H = 1200 ext{ K}$ (both rejecting at $T_C = 300 ext{ K}$), what does this illustrate about efficiency gains?