What is the primary way a catalyst accelerates a chemical reaction?
Answer
Offering an alternative reaction mechanism with a lower energy barrier
The fundamental action of a catalyst is providing a different pathway for the transformation. This new route involves a series of steps, each having a lower energy barrier compared to the single high barrier of the uncatalyzed path.
#Videos
Activation Energy and Catalysts - YouTube
Related Questions
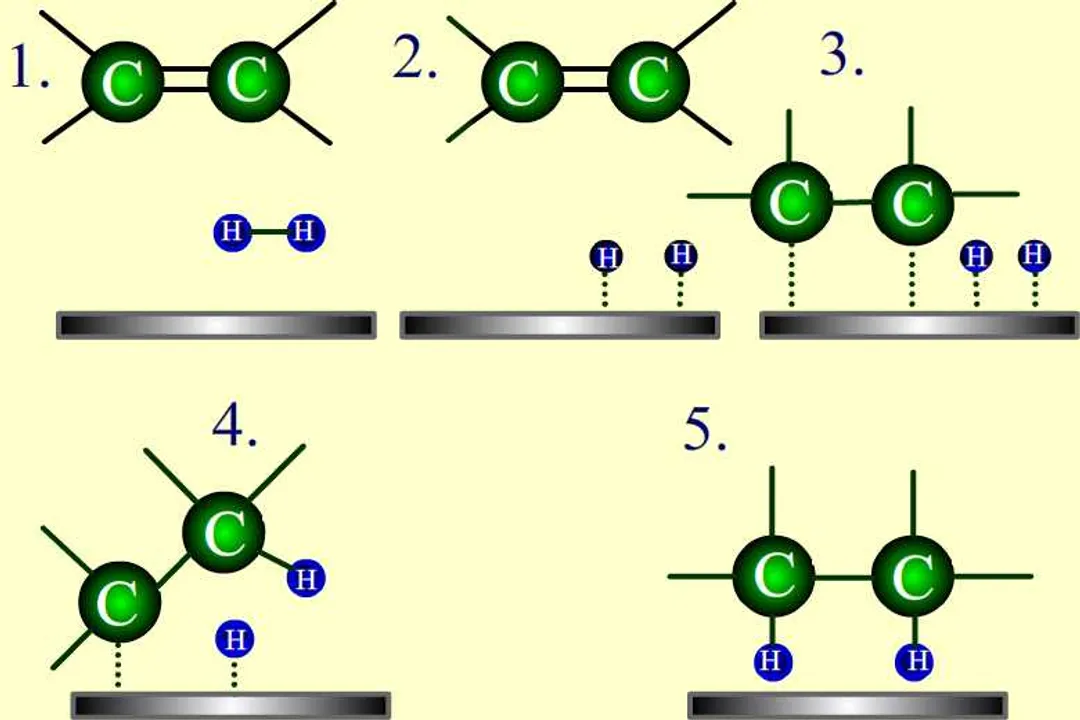
What does activation energy ($E_a$) represent in a chemical reaction?What is the primary way a catalyst accelerates a chemical reaction?Does the presence of a catalyst change the inherent thermodynamics ($\Delta H$) of a reaction?On an energy profile diagram, what is true regarding the reactant and product energy levels for both catalyzed and uncatalyzed reactions?According to the Arrhenius equation structure ($k = A \cdot e^{-E_a / RT}$), what effect does a decrease in $E_a$ have on the rate constant ($k$)?What must be true about the phase of the catalyst relative to the reactants in heterogeneous catalysis?How does a catalyst affect the position of a chemical equilibrium?What critical condition must be met for a substance to be classified as a catalyst?What is the key advantage provided by using catalysts like iron in the Haber-Bosch process?What term describes specialized biological catalysts that function effectively under mild conditions like body temperature and neutral pH?