What does activation energy ($E_a$) represent in a chemical reaction?
Answer
The minimum energy required for reactants to successfully collide and form products
Activation energy is the initial energy investment or hurdle that molecules must overcome, often visualized as the height of a hill leading to the transition state. Only molecules possessing kinetic energy equal to or greater than this threshold can react.
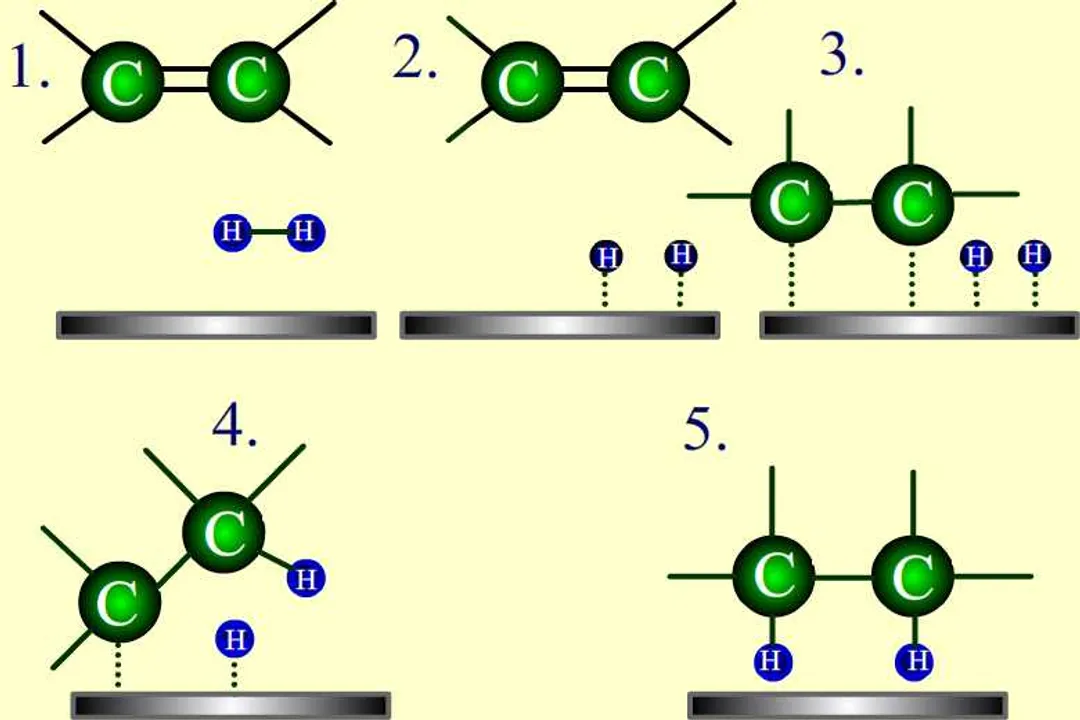
#Videos
Activation Energy and Catalysts - YouTube
Related Questions
What does activation energy ($E_a$) represent in a chemical reaction?What is the primary way a catalyst accelerates a chemical reaction?Does the presence of a catalyst change the inherent thermodynamics ($\Delta H$) of a reaction?On an energy profile diagram, what is true regarding the reactant and product energy levels for both catalyzed and uncatalyzed reactions?According to the Arrhenius equation structure ($k = A \cdot e^{-E_a / RT}$), what effect does a decrease in $E_a$ have on the rate constant ($k$)?What must be true about the phase of the catalyst relative to the reactants in heterogeneous catalysis?How does a catalyst affect the position of a chemical equilibrium?What critical condition must be met for a substance to be classified as a catalyst?What is the key advantage provided by using catalysts like iron in the Haber-Bosch process?What term describes specialized biological catalysts that function effectively under mild conditions like body temperature and neutral pH?