How do intermolecular forces influence boiling points?
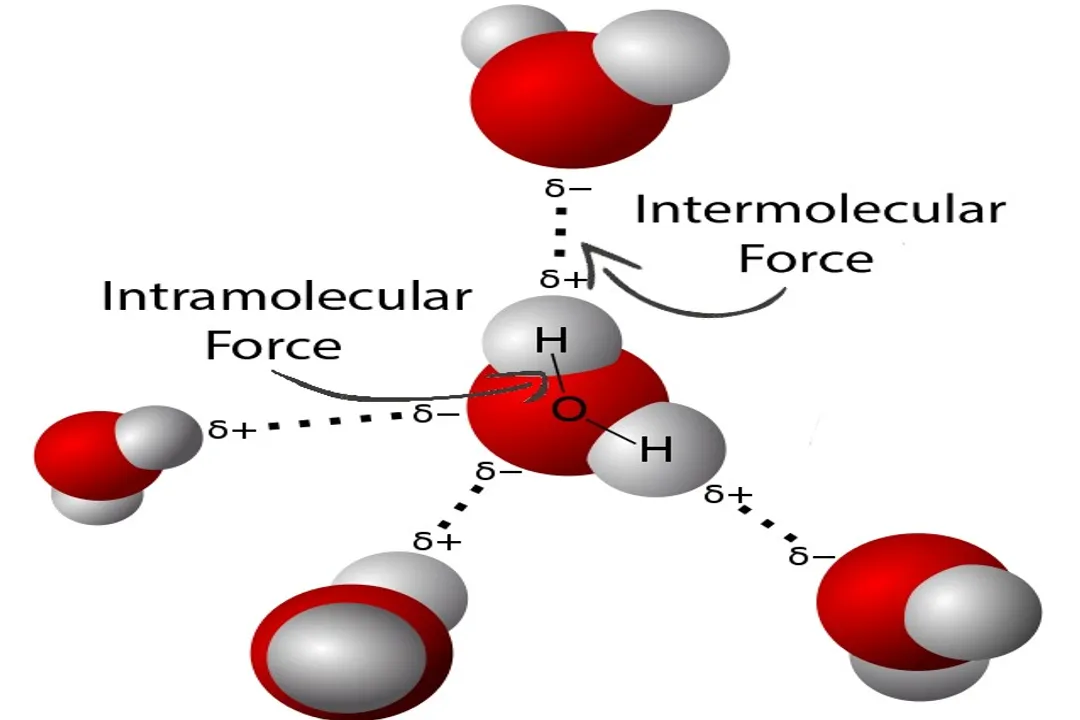
The temperature at which a substance transitions from a liquid to a gas—its boiling point ()—is a direct consequence of the energy required to break the attractive forces holding its molecules together in the condensed liquid phase. These forces, known as intermolecular forces (), are electrostatic in nature and are far weaker than the intramolecular forces, like covalent bonds, that hold the atoms within a molecule together. To reach the boiling point, molecules must absorb enough thermal energy to overcome these attractions and enter the independent, gaseous state. A substance with strong will have molecules clinging tightly together, demanding more kinetic energy (i.e., a higher temperature) to separate them. Conversely, weaker mean the liquid has a higher tendency to evaporate, resulting in a higher vapor pressure and, therefore, a lower boiling point.
# IMF Hierarchy
To compare the boiling points of different substances, chemists establish a hierarchy of strength. While all substances experience some form of attraction, these forces exist on a spectrum, generally ordered from strongest to weakest as follows: ionic forces, hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
Ionic Forces represent the attraction between fully charged ions, such as and . Because these involve complete charges, they are the strongest forces, reflected in the exceptionally high melting and boiling points of ionic solids.
Hydrogen Bonding is the next strongest attraction for neutral molecules. It is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is directly bonded to a highly electronegative atom: Nitrogen (), Oxygen (), or Fluorine (). The small size of the hydrogen atom allows the positive end of the bond dipole to approach the lone pair of electrons on the neighboring electronegative atom very closely, leading to a powerful electrostatic attraction.
Dipole-Dipole Interactions exist between any molecules that possess a net dipole moment—a permanent imbalance of charge due to polar covalent bonds that do not cancel out across the molecule. These are significant but generally weaker than hydrogen bonds because the partial charges involved are less extreme than the charge separation in an -bond.
London Dispersion Forces (), also known as van der Waals dispersion forces, are the weakest of the common and are present in every molecule, whether polar or nonpolar. They arise from instantaneous, temporary fluctuations in electron distribution around an atom or molecule, creating transient dipoles that induce dipoles in neighbors.
# Dispersion Dominance
For nonpolar molecules—such as alkanes, which only contain bonds— are the only attractive forces present, making their boiling points entirely dependent on these fluctuations. The strength of is governed by two main structural factors: molecular size (or mass) and surface area.
Larger molecules, which possess more electrons and greater mass, have higher boiling points than smaller molecules of the same kind. This is because the larger electron clouds are more polarizable—meaning their electron distributions are more easily distorted to create those temporary dipoles. For instance, comparing noble gases, (), a larger atom, boils at a significantly higher temperature () than () (). The increase in boiling point along a homologous series of alkanes (like ) follows this trend of increasing mass and size, which enhances the cumulative effect of .
The second structural influence on is the molecule's shape, which dictates the effective surface area available for intermolecular contact. Molecules that are linear or extended have a greater surface area for interaction than those that are compact or spherical. This is clearly demonstrated when comparing structural isomers, which have the same mass but different shapes. () is a relatively linear chain, allowing for greater contact area between molecules, giving it a boiling point of . Its highly branched isomer, (), is almost spherical, resulting in minimal surface area for interaction, thus its boiling point is much lower, at .
When comparing compounds where the type differs, the situation becomes a balancing act between the inherent strength of the force and the overall size/polarizability of the molecule. It is crucial to understand that are present in all molecules, meaning even a highly polar molecule is still experiencing dispersion forces.
Consider this illustrative comparison involving molecules of very similar molecular weight (around ):
| Molecule | Primary IMF Type | Molecular Weight () | Boiling Point () | Rationale |
|---|---|---|---|---|
| (Diethyl Ether) | Dipole-Dipole ( present) | $74$ | $34.6$ | Polar, but only dipole-dipole attraction is significant. |
| (1-Butanol) | Hydrogen Bonding ( present) | $74$ | $117.7$ | Presence of group raises substantially. |
| (Pentane) | London Dispersion Forces Only | $72$ | $36.1$ | Nonpolar, relies solely on LDFs, comparable to the ether. |
| (Cyclopentene) | London Dispersion Forces Only | $70$ | $44$ | Nonpolar, but slightly larger/more surface area than pentane [cite: 3, implied comparison]. |
What this table highlights is a subtle point often glossed over: for substances of similar size, the type of dominates the . However, when comparing a large nonpolar molecule to a smaller polar one, the massive increase in due to size can sometimes overcome the dipole-dipole interaction of the smaller molecule. For example, a larger alkane (, nonpolar) can have a boiling point of , potentially higher than a smaller molecule capable only of weak dipole-dipole forces, because its sheer size generates overwhelming dispersion forces.
# Polar Attractions
When comparing molecules that have dipoles, the strength of the interaction is related to the magnitude of the dipole moment (). In a series of molecules with similar molar masses, the one with the largest dipole moment will have the highest boiling point due to stronger dipole-dipole attractions. For instance, when comparing (a ketone), (an ether), and (a nonpolar alkane), all having masses around : has the largest dipole moment (), leading to the highest (), while (very small ) has the lowest ().
This comparison underscores that even for polar molecules, the (which scale with size/mass) are still contributing factors, but the permanent dipole dictates the relative ordering when masses are similar.
# Hydrogen Bond Strength
The influence of hydrogen bonding is so pronounced that it often causes a massive deviation in boiling point trends compared to what molecular mass alone would predict. In the hydrides of Group 15, 16, and 17 elements, as you move down the group, mass increases and boiling points generally rise as expected due to stronger . However, the lightest members—, , and —have boiling points over higher than predicted based on this trend, solely because of -bonding.
(, ) boils at , whereas its heavier neighbor, (, ), boils at a frigid because it lacks strong -bonds.
The exceptional strength of -bonding in water stems from its ability to maximize the number of these interactions. Each water molecule can act as a donor (via its two bonds) and an acceptor (via its two lone pairs), resulting in an average of four -bonds per molecule in its network. This results in an open, cagelike, three-dimensional structure in ice. () can also -bond, but on average, it forms only two -bonds per molecule (one donor, two acceptors), leading to a significantly lower boiling point () than water, despite having a greater molecular mass ().
When assessing -bonding capabilities, one must check for both a hydrogen donor ( attached to , , or ) and an -bond acceptor (a lone pair on , , or ). For example, () can accept -bonds but cannot donate them, leaving it with weaker and dipole-dipole attractions, resulting in a of .
# Shape vs. Size in Phase Transitions
While boiling points are primarily concerned with overcoming attractions in the liquid state, melting points () bring molecular packing into sharp focus. Melting requires enough energy to break the highly ordered crystalline lattice, and how efficiently molecules fit together dictates the lattice strength.
For a set of structural isomers—molecules with the identical formula but different connectivity and shape—the type is the same, so the boiling point trend is dictated by surface area (). A linear molecule will generally have a higher than its branched counterpart because the greater surface area leads to stronger collective .
However, the melting point difference between these same isomers can be much more dramatic because of packing efficiency. Spherical molecules, like (), can pack into a crystal lattice surprisingly well, giving it an exceptionally high (), only slightly lower than its (). In contrast, its linear isomer, (), boils slightly higher () but melts much lower (). This is an excellent example of how the process matters: boiling separates molecules, whereas melting requires the molecules to adopt a rigid, ordered geometric structure; thus, molecular shape is disproportionately critical for melting points.
Another factor that drastically affects but has little effect on is the presence of impurities. Impurities disrupt the perfect crystal lattice arrangement, making the cumulative intermolecular interactions within the solid weaker, which consequently lowers and broadens the observed melting point. The boiling point, reflecting the less structured liquid state, is not as sensitive to these minor packing imperfections.
# External Conditions
Beyond the inherent nature of the molecules themselves, the environment plays a role in determining the actual boiling point value observed. The definition of boiling is when the vapor pressure equals the surrounding pressure. If the external atmospheric pressure is reduced, less energy is needed for the vapor pressure to match it, causing the liquid to boil at a lower temperature. This is why water boils below at high altitudes.
Understanding the interplay between these forces—from the powerful, localized charge of an ionic bond to the subtle, fluctuating attraction between two noble gas atoms—is key to predicting the physical behavior of any substance. The boiling point serves as the simplest macroscopic indicator of these microscopic attractions: stronger forces demand a greater input of thermal energy to achieve a phase transition to gas.
Related Questions
#Citations
2.11: Intermolecular Forces and Relative Boiling Points (bp)
The Four Intermolecular Forces and How They Affect ...
Understanding How Intermolecular Forces Affect Boiling ...
13.1 Intermolecular Forces
Boiling & Melting Points
Effects of Intermolecular Forces
How do intermolecular forces affect the boiling point?
Can someone please explain intermolecular forces and ...

