What is the thermodynamic objective achieved when a solid forms into a stable crystal lattice?
Answer
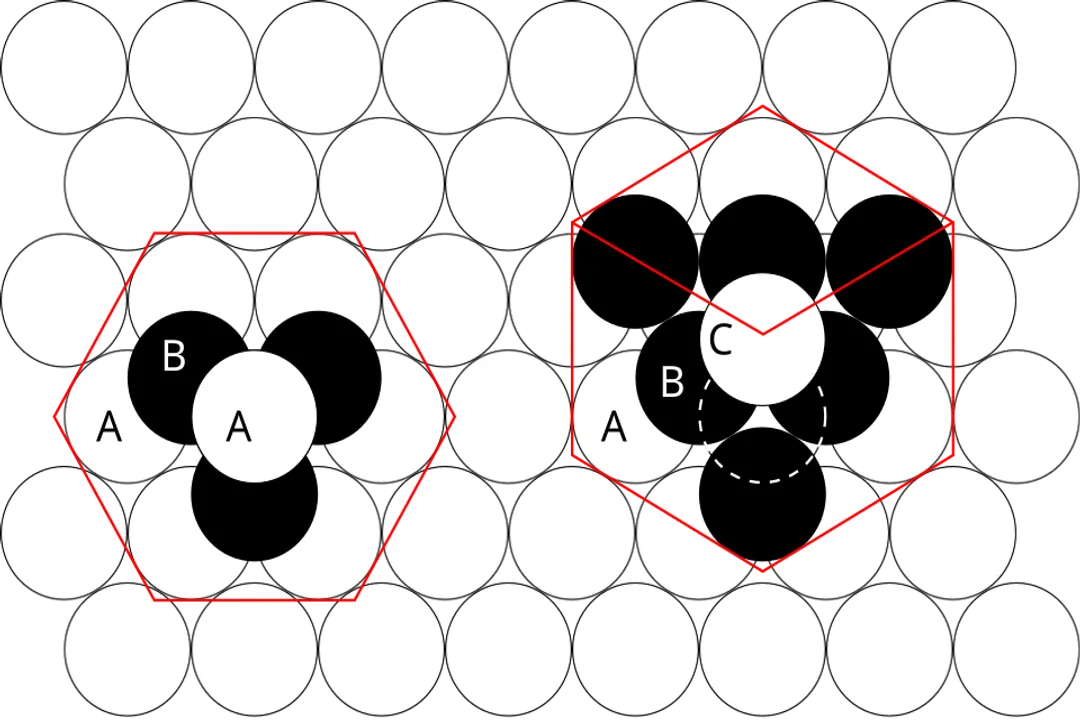
Achieving the lowest possible potential energy state.
The formation of a crystal lattice represents a transition to the most energetically favorable three-dimensional pattern, which corresponds to thermodynamic stability.
Related Questions
What fundamentally defines the arrangement of matter in a crystal lattice?What is the primary factor governing the structure of ionic compounds?What dictates the lattice geometry in materials held together by covalent bonds, such as diamond?What arrangement tendency do metallic solids often exhibit due to less directional bonding forces?What is the thermodynamic objective achieved when a solid forms into a stable crystal lattice?What is the packing efficiency achieved by the most efficient sphere packing structures, FCC and HCP?What term describes the 14 unique ways points can be periodically arranged in three dimensions?In ionic crystals, what critical ratio dictates the resulting geometry and coordination number?What structural outcome is typically observed if a material is cooled extremely slowly from the melt?What state results when rapid cooling (quenching) traps atoms before they can reach the lowest energy arrangement?